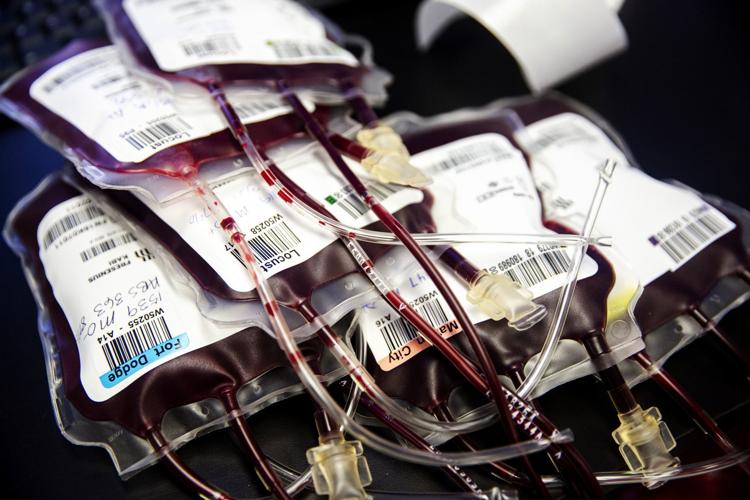
Thinking about a controversial infusion of “young blood,” now offered in Omaha, as an anti-aging boost? The FDA has issued a warning saying such treatments are “potentially harmful.”
Infusing a young person’s blood plasma into an older person to ward off the effects of aging and disease has no proven clinical benefit and could be risky, the U.S. Food and Drug Administration said in a statement Tuesday.
A company in January began offering infusions of so-called “young blood” in Omaha, and five other cities, through its website. The Florida-based company, Ambrosia Health, listed the price of infusions from people ages 16 to 25 at $8,000 a liter or $12,000 for two, payable via PayPal. It’s available to people 30 or older.
Jesse Karmazin, the company’s founder, could not be reached immediately Tuesday for comment.
He said in an interview with The World-Herald last month that the yet-to-be published results of a clinical trial conducted by the company, which evaluated biomarkers of aging before and after treatment, found “real, objective improvements.”
But a number of experts who work in the field have said there is no scientific evidence in humans to show that infusions of plasma from young people have an effect on aging. Now the FDA is echoing that concern.
FDA officials said the agency had become aware of establishments in several states offering the infusions to treat conditions ranging from normal aging and memory loss to serious diseases including Parkinson’s disease, multiple sclerosis, Alzheimer’s disease, heart disease and post-traumatic stress disorder.
“There is no proven clinical benefit of infusion of plasma from young donors to cure, mitigate, treat, or prevent these conditions, and there are risks associated with the use of any plasma product,” wrote FDA Commissioner Scott Gottlieb and Peter Marks, director of FDA’s Center for Biologics Evaluation and Research.
The officials said none of the plasma treatments has gone through the rigorous testing the agency normally requires. “As a result, the reported uses of these products should not be assumed to be safe or effective,” they wrote.
While the U.S. blood supply and infusions of blood products are generally considered safe, there are known risks, including allergic reactions ranging from mild to life-threatening, lung injury, circulatory overload and new diseases that haven’t yet been detected.
Plasma is the liquid portion of blood that contains proteins to help blood clot. Plasma infusion is an approved use by the FDA in trauma settings or in patients whose blood doesn’t coagulate.
The use of blood products is an active area of aging research. Karmazin said in his earlier interview that work on what’s known as parabiosis in mice is the scientific basis for Ambrosia’s treatments. In those studies, researchers who surgically joined the blood systems of young and old mice observed some positive effects on the health of aging mice.
FDA approval of a treatment typically requires human trials before companies can make a specific health claim about a product. So far, only one small clinical trial in humans has been published. The main conclusion was that the treatment was safe.
Karmazin said last month that Ambrosia’s clinical trial, which treated about 100 patients with one dose of plasma each, wrapped up about a year ago. He said the company had presented some results at a conference and would be publishing more soon. “We have quite a bit of data to show this works,” he said last month.
The FDA officials, however, “strongly discouraged” consumers from pursuing the therapy outside clinical trials under appropriate institutional review and regulatory oversight.
“We’re concerned that some patients are being preyed upon by unscrupulous actors touting treatments of plasma from young donors as cures and remedies,” Gottlieb and Marks wrote. “Such treatments have no proven clinical benefits for the uses for which the clinics are advertising them, and are potentially harmful.”
It’s not clear whether any of the treatments have been administered in Omaha.
The Omaha location was among several new ones that opened at the beginning of the year. Karmazin said a doctor who has experience with transfusions reached out to him and said he wanted to start treating patients.
Karmazin declined to give the location of the Omaha clinic. The company no longer publicly lists the locations, he said, because they have attracted “unwanted visitors.”
He also declined to identify the physician providing the treatments for the privacy of his patients. That information, he said, is provided when patients sign up for treatment.
Bloomberg contributed to this report.